-
게시판-관련소식
뉴스레터 2021년 5월호
2021-06-02 by ISPE Korea
-
MFDS 소식
-
생물학적제제 제조소 생물안전 평가지침(공무원지침서) 개정
-
생물학적제제 제조용 생물체 취급에 따른 위험으로부터 안전하게 제조가 이루어질 수 있도록 '생물학적제제 제조소 생물안전 평가지침(공무원지침서)'을 개정하였음을 알려드립니다.
-
'바이오의약품 사전 GMP 평가 지침(공무원 지침서)' 개정
-
'바이오의약품 사전 GMP 평가 지침(공무원지침서)'을 코로나19 상황 및 첨단재생바이오법 시행 등에 따라 개정하였음을 알려드립니다.
-
-
GMP 가이드라인
-
Draft Guidance for Industry: Cosmetic Good Manufacturing Practices
-
This document provides guidance to industry and other stakeholders (e.g., consumer interest groups, academia, other regulatory groups) on FDA’s current thinking concerning what constitutes Good Manufacturing Practices (GMPs) for cosmetics.
-
Non-Penicillin Beta-Lactam Drugs: A CGMP Framework for Preventing Cross-Contamination
-
This guidance describes the importance of implementing manufacturing controls to prevent cross-contamination of finished pharmaceuticals and active pharmaceutical ingredients (APIs) with non-penicillin beta-lactam drugs.
-
-
ISPE 국내외 소식
-
ISPE KOREA 온라인교육 (5월)
-
2021.05.25 (13:00~14:50)
Pharmaceutical Development Cycle from Idea to Business and GMP Compliances (바이러스기반 바이오의약품 개발 및 제품화 준비 과정)
강사: 박기랑 (주식회사 씨드모젠 / 대표이사)
-
2021.05.25 (15:00~15:50)
Lab Control
김부선 (한국에프디시법제학회 / GMP부문장)
-
Upcoming Conferences
-
2021 ISPE Global Pharmaceutical Regulatory summit
16 June 2021
Virtual Summit
Theme: Quality Risk Management Ensuring Shared Responsibility across Key Stakeholders
-
2021 ISPE Asia Pacific Pharmaceutical Mfg Conference
17-18 June 2021
Virtual Conterence
Theme: Sustainable Implementation of Quality Risk Management
-
Guidance Documents
-
Good Practice Guide: Maintenance 2nd Edition
Published: January 2021
Pages: 032
Member Price: $295.00
Non-Member Price: $595.00
-
APQ Guide: Corrective Action & Preventive Action (CAPA) System
Published: November 2020
Pages: 136
Member Price: $215.00
Non-Member Price: $515.00
-
ISPE Publishes ISPE GAMP RDI Good Practice Guide: Data Integrity by Design
Published: November 2020
Pages: 176
Member Price: $395.00
Non-Member Price: $695.00
-
Guide: Cleaning Validation Lifecycle - Applications, Methods, & Controls
Published: August 2020
Papes: 236
Member Price: $495.00
Non-Member Price: $795.00
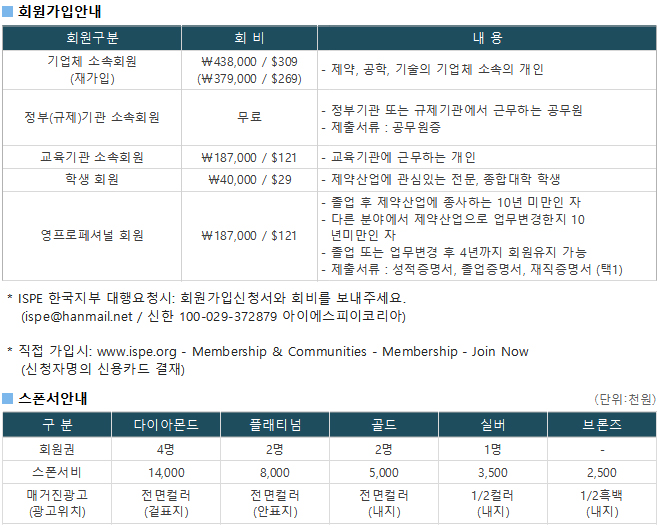
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
