-
게시판-관련소식
뉴스레터 2021년 9월호
2021-09-27 by ISPE Korea
-
MFDS 소식
-
임상시험 안전성 정기보고 온라인 정책설명회 발표자료
-
임상시험용의약품 최신 안전성 정보 보고(DSUR) 정책설명회 발표자료 및 발표영상 공유드립니다.
-
(GRP-MaPP-허가업무-02) 일반의약품 허가사항 심사시 일반적 고려사항(6개정) 개정
-
이 지침서는 일반의약품 심사시 일반적 고려사항에 대한 식품의약품안전처 관련 부서 담당 직원의 업무처리를 위한 것입니다.
-
-
GMP 가이드라인
-
PET Drugs--Current Good Manufacturing Practice(CGMP); Small Entity Compliance Guide
-
This guidance is intended to help small businesses understand and comply with FDA's organ-specific labeling regulation for over-the-counter(OTC) internal analgesic, antipyretic, and antirheumatic(IAAA) drug products.
-
Process Validation: General Principles and Practices
-
This guidance outlines the general principles and approaches that FDA considers appropriate elements of process vlidation for the manufacture of human and animal drug and biological products, including active pharmaceutical ingredients (APIs or durg substances), collectively referred to in this guidance as drugs or products.
-
-
ISPE 국내외 소식
PHARMACEUTICAL ENGINEERING 9-10 (한글판) 진행중
- A Vision for ICH Q12: CURRENT EXPERIENCE, FUTURE PERSPECTIVES
- DISTANT ASSESSMENTS, AUDITS, AND REGULATORY GUIDANCE
- PHARMACEUTICAL CLEANROOM DESIGN and ISO 14644-16
- TEMPERATURE AND HUMIDITY REQUIREMENTS in Pharmaceutical Facilities
- SAFEGUARDING VIAL CONTAINER CLOSURE INTEGRITY: A Systematic Approach
* 국제회원에게 책자 보내드립니다.
-
ISPE KOREA 9월 온라인교육 진행완료
-
2021.09.14 (13:00~15:00)
EU GMP; Eudralex Vol,4 Annex 1의 이해
오성창 (씨티씨바이오 / 상무)
-
2021.9.14 (15:30~17:50)
T5 Validation
안도영 (아이비에스/대표이사)
-
Upcoming Conferences
-
2021 ISPE Biotechnology Conference & Workshop
22-24 September 2021
Virtual Conference & Workshop
-
2021 ISPE Annual Meeting & Expo
21 October - 3 November 2021
Boston, MA
Theme: Agility. Collaboration. Innovation
-
Guidance Documents
Good Practice Guide: IMP Reverse Logistics
Published: August 2021
Pages: 56
Member Price: $295.00
Non-Member Price: $595.00
-
Good Practice Guide: Maintenance 2nd Edition
Published: January 2021
Pages: 032
Member Price: $295.00
Non-Member Price: $595.00
-
APQ Guide: Corrective Action & Preventive Action (CAPA) System
Published: November 2020
Pages: 136
Member Price: $215.00
Non-Member Price: $515.00
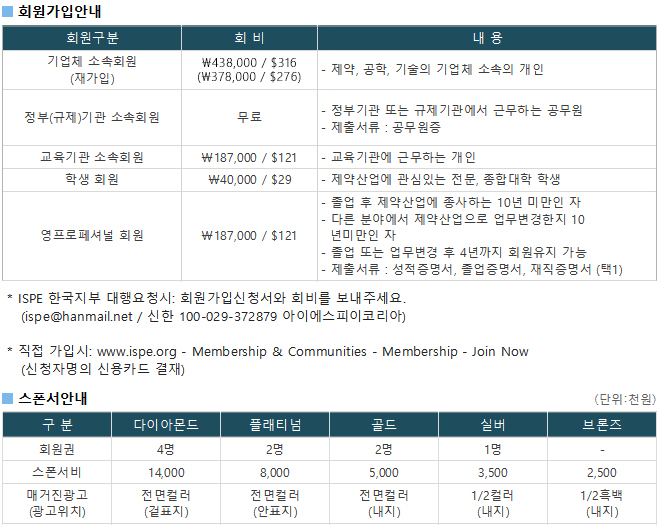
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
