-
게시판-관련소식
뉴스레터 2021년 10월호
2021-10-28 by ISPE Korea
-
MFDS 소식
-
'의약품 임상시험 등 종사자 교육 및 교육실시기관 지정에 관한 규정' 일부개정고시안
-
식품의약품안전처 공고 제2021-485호
개정이유: 임상시험 종사자의 교육 이수시간 및 이수방법을 합리적으로 개선하는 등 현행제도의 운영상 나타난 일부 미비점을 개선, 보완하여 교육이수의 편의를 도모하고자 함.
-
'의약품등 품목별 사전 GMP 평가 운영지침(공무원 지침서)' 개정
-
의약품등의 품목별 사전 GMP 평가 관련 서류검토 요건, 현장실태조사 대상 및 조사기간 등 세부사항을 정함으로써 의약품등의 제조판매, 수입품목 (변경)허가, 신고 대상 품목의 제조 및 품질관리기준(GMP) 적합여부 평가 및 판정에 적정을 기하기 위함.
-
-
GMP 가이드라인
-
The Use of Mechanical Dissolution Apparatus 1 and 2-Current Good Manufacturing Practice(CGMP): Guidance for Industry
-
This guidance is intended to aid drug manufacturers (including ancillary testing laboratories) in calibrating U.S. Pharmacopeia(USP) Dissolution Apparatus 1 and 2 to help assure that critical parameters associated with the dissolution apparatus meet certain mechanical calibration (MC) tolerances.
-
PET Drug Products - Current Good Manufacturing Practice (CGMP)
-
This guidance is intended to help positron emission tomography (PET) drug producers better understand FDA's thinking concerning compliance with the current good manufacturing practice (CGMP) regulations.
-
ISPE 국내외 소식
-
ISPE KOREA 온라인교육 (11월)
-
2021.11.16 (13:00~15:00)
Pharmaceutical Quality System (ICH Q10)
오성창 공장장 (씨티씨바이오 / 홍천사업장)
PHARMACEUTICAL ENGINEERING (9-10 한글판)
- A Vision for ICH Q12: CURRENT EXPERIENCE, FUTURE PERSPECTIVES
- DISTANT ASSESSMENTS, AUDITS, AND REGULATORY GUIDANCE
- PHARMACEUTICAL CLEANROOM DISIGN and ISO 14644-16
- TEMPERATURE AND HUMIDITY REQUIREMENTS in Pharmaceutical Facilities
- SAFEGUARDING VIAL CONTAINER CLOSURE INTEGRITY: A Systematic Approach
* 국제 멤버쉽회원에게 책자를 보내드립니다 (10월말경)
-
Upcoming Conferences
-
2021 ISPE Annual Meeting & Expo
31 October - 3 November 2021
Boston, MA
Theme: Agility. Collaboration. Innovation
-
2021 ISPE Pharma 4.0 & Annex 1 Conference
8 - 9 December 2021
Vienna, Austria
2022 ISPE Facilities of the Future Conference
1 - 2 February 2022
North Bethesda, MD
2022 Aseptic Conference
14 - 15 March 2022
North Bethesda, MD
Theme: Where Regulatory Guidance & Implemention Meet
-
Guidance Documents
-
Good Practice Guide: Maintenance 2nd Edition
Published: January 2021
Pages: 032
Member Price: $295.00
Non-Member Price: $595.00
Good Practice Guide: Knowledge Management in the Pharmaceutical Industry
Published: May 2021
Pages: 160
Member Price: $295.00
Non-Member Price: $595.00
APQ Guide: Management Responsibilities & Review (MRR)
Published: July 2021
Pages: 162
Member Price: $215.00
Non-Member Price: $515.00
-
Good Practice Guide: IMP Reverse logistics
Published: August 2021
Papes: 56
Member Price: $295.00
Non-Member Price: $595.00
GAMP Good Practice Guide: Enabling Innovation
Published: September 2021
Pages: 102
Member Price: $295.00
Non-Member Price: $595.00
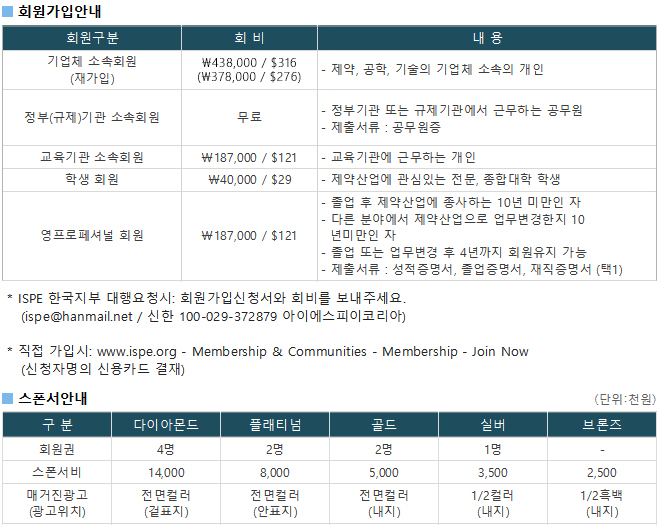
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
