-
게시판-관련소식
뉴스레터 2021년 11월호
2021-11-23 by ISPE Korea
-
MFDS 소식
-
2021년 하반기 의약품 허가업무 온라인 설명회 자료
-
심각한 중증질환 또는 희귀질환을 치료하기 위한 목적으로 사용되는 의약품 중 총리령으로 정하는 의약품에 대하여 품목 조건부 허가를 할 수 있는 법적 근거 마련. [약사법] 제35조 개정 ('21.7.20 개정, '22.1.21 시행)
-
바이오의약품 전문수탁 제조업체 GMP평가 절차(공무원지침서) 개정
-
국내 바이오의약품 수출지원, 글로벌 백신 허브 구축 등을 위하여 '바이오의약품 전문수탁 제조업체 GMP 평가 절차'를 개정하였음을 알려드리니 참고하시기 바랍니다.
[의약품 제조 및 품질관리에 관한 규정] 일부개정 고시
-
-
GMP 가이드라인
-
Pharmaceutical Components at Risk for Melamine Contamination: Guidance for Industry
-
This guidance is intended to alert pharmaceutical manufacturers of finished products, pharmacy compounders, repackers, and suppliers to the potential risk of melamine contamination in pharmaceutical components.
-
Current Good Manufacturing Practice for Phase 1 Investigational Drugs
-
This guidance is intended to assist in applying current good manufacturing practice (CGMP) required under section 501(a)(2)(B) of the Federal Food, Drug, and Cosmetic Act (FD&C Act) in the manufacture of most investigational new drugs (IND) used in phase 1 clinical trials.
-
-
ISPE 국내외 소식
-
Pharmaceutical Engineering (11-12월호 한글판)
-
November-December 2021 / Volume 41, Number 6
- Ai's Promise for ATMPS
- Supporting Scalable Cell Therapy Using Allogeneic Workflows
- Personalized Medicine: The Industry's Future
- Changing Lives with Gene Therapies
- Today's Pharma and Biotech Projects: A Phased Approach
- ISPE Launches ATMP Community of Practice
- 2021 ISPE Biotechnology Conference and Workshop Cell and Gene Therapies Star in Conference Keynotes
- Implementing Low ph Viral Inactivation in a Continuous Process
-
Upcoming Conferences
ISPE Expert Xchange: Risk Based Decision Making: Advancing the Integration of QRM & KM
2 December 2021
Virtual Session
-
2021 ISPE Pharma 4.0 & Annex 1 Conference
8 - 9 December 2021
Vienna, Austria
-
2022 ISPE Facilities of the Future Conference
1 - 2 February 2022
North Bethesda, MD
-
Guidance Documents
-
APQ Guide: Management Responsibilities & Review (MRR)
Published: November 2020
Pages: 162
Member Price: $215.00
Non-Member Price: $515.00
-
Good Practice Guide: IMP Reverse Logistics
Published: August 2021
Pages: 56
Member Price: $295.00
Non-Member Price: $595.00
-
GAMP Good Practice Guide: Enabling Innovation
Published: September 2021
Papes: 102
Member Price: $295.00
Non-Member Price: $595.00
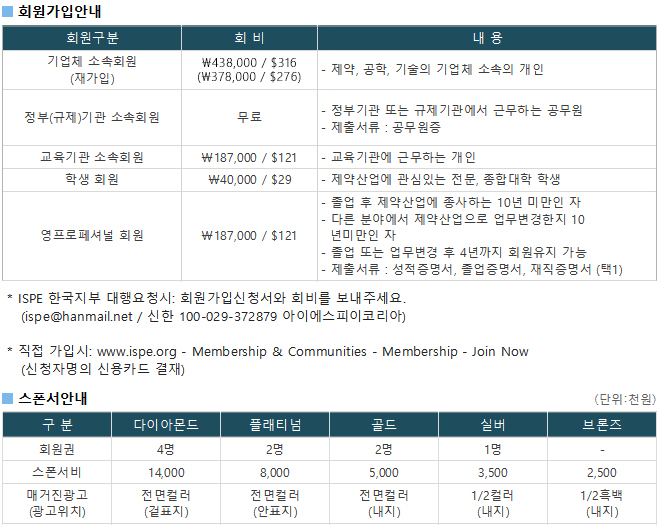
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
