-
게시판-관련소식
뉴스레터 2021년 12월호
2021-12-15 by ISPE Korea
-
MFDS 소식
-
「대한민국약전」 일부개정고시(안) 행정예고
-
「약사법」 제51조제1항에 따른 「대한민국약전」 (식품의약품안전처 고시 제2021-101호, 2021.12.02)을 일부 개정함에 있어 국민에게 미리 알려 의견을 수렴하고자 그 취지, 개정이유 및 주요내용을 「행정절차법」 제46조에 따라 다음과 같이 공고합니다.
-
2021년 의약품심사 온라인 설명회 자료
-
2021년 하반기 의약품심사 온라인 설명회 자료를 게재합니다.
-
-
GMP 가이드라인
-
Computerized Systems Used in Clinical Investigations
-
This document provides to spnsors, contract research organizations (CROs), data management centers, clinical investigators, and institutional review boards (IRBs), recommendations regarding the use of computerized systems in clinical investigations.
-
Testing of Glycerin for Diethylene Glycol
-
This guidance is intended to alert pharmaceutical manufacturers, pharmacy compounders, repackers, and suppliers to the potential public health hazard of glycerin contaminated with diethylene glycol (DEG), a poison.
-
-
ISPE 국내외 소식
-
ISPE KOREA 온라인교육
-
2022.01.11 (13:00~15:00)
Pharmaceutical Water System
강사: 안도영 (주식회사 아이비에스 / 대표이사)
PHARMACEUTICAL ENGINEERING
-
November-December 2021 / Volume 41, Number 6
AI'S PROMISE FOR ATMPS
SUPPORTING SCALABLE CELL THERAPY USING ALLOGENEIC WORKFLOWS
PERSONALIZED MEDICINE: The Industry's Future
CHANGING LIVES WITH GENE THERAPIES
TODAY'S PHARMA AND BIOTECH PROJECTS: A Phased Approach
ISPE LAUNCHES ATMP COMMUNITY OF PRACTICE
2021 ISPE BIOTECHNOLOGY CONFERENCE AND WORKSHOP CELL AND GENE THERAPIES STAR IN CONFERENCE KEYNOTES
IMPLEMENTING LOW PH VIRAL INACTIVATION IN A CONTINUOUS PROCESS
* 2022.01 국제회원분들께 배송드릴 예정입니다.
-
Upcoming Conferences
-
ISPE Expert Xchange: Risk Based Decision Making: Advancing the Integration of QRM & KM
18 January 2022
Virtual Session
-
2022 ISPE Facilities of the Future Conference
1-2 February 2022
North Bethesda, MD
2022 ISPE Aseptic Conference
14-15 March 2022
North Bethesda, MD
Theme: Where Regulatory Guidance & Implementation Meet
2022 ISPE Europe Annual Conference
24-27 April 2022
Madrid, Apain
-
Guidance Documents
-
Good Practice Guide: Good Engineering Practice 2nd Edition
Published: October 2021
Pages: 244
Member Price: $295.00
Non-Member Price: $595.00
-
GAMP Good Practice Guide: Enabling Innovation
Published: September 2021
Pages: 102
Member Price: $295.00
Non-Member Price: $595.00
-
Good Practice Guide: IMP Reverse Logistics
Published: August 2021
Pages: 56
Member Price: $295.00
Non-Member Price: $595.00
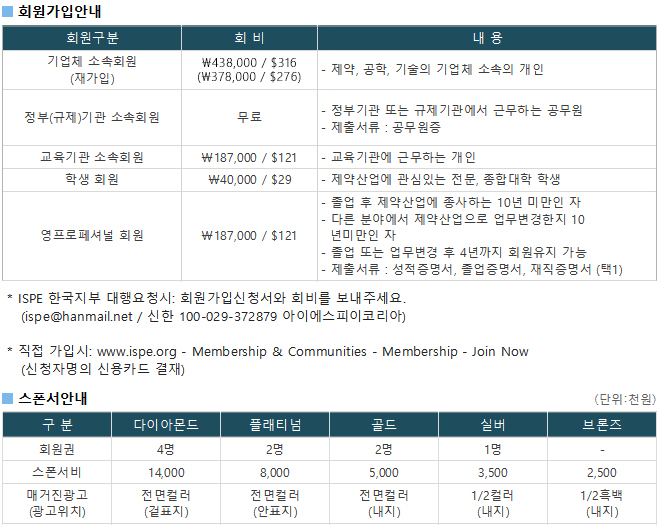
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
