-
게시판-관련소식
뉴스레터 2022년 6월호
2022-06-24 by ISPE Korea
-
MFDS 소식
-
'의약품등 회수에 관한 규정' 일부개정고시
-
회수 의무자는 의약품등의 회수를 완료한 경우 지방청장에게 회수종료신고서를 제출하여야 하고, 지방청장은 회수 적절 이행여부 확인을 위한 판매처의 10%이상을 선정하여 회수 점검을 실시한후 회수종료여부를 평가하여야 함. 공중보건위기상황 등 식약처장이 필요하다고 인정하는 경우에는 판매처 수와 점검에 따른 행정비용 등을 고려하여 효율적인 점검이 가능하도록 회수점검 대상을 조정하여 제도운영의 유연성을 제고하고자 함.
GRP-MaPP-심사기준-12_생물학적동등성시험 계획(변경)승인 검토서 작성기준(10개정)
'GRP-Mapp-심사기준-12_생물학적동등성시험 계획(변경)승인검토서 작성기준(10개정)'을 붙임과 같이 개정하여 게시하오니 관 련업무에 참고하시기 바랍니다.
-
-
GMP 가이드라인
-
Quality Considerations for Continuous Manufacturing
-
This guidance provides information regarding FDA's current thinking on the quality considerations for continuous manufacturing of small molecule, solid oral drug products that are regulated by the Center for Drug Evaluation and Research (CDER). ~
-
Mixing, Diluting, or Repackaging Biological Products Outside the Scope of an Approved Biologics License Application Guidance for Industry
-
This guidance sets forth FDA's policy regarding the mixing, diluting, and repackaging of certain types of biological products that have been licensed under section 351 of the Public Health Service Act (PHS Act) when such activities are not within the scope of the product's approved biologics license application (BLA) as described in the approved labeling for the product. ~
-
-
ISPE 국내외 소식
ISPE KOREA 교육회원 모집
* 가입대상: 제약 및 Biopharma 관련업계 종사하는 모든 분
* 회원혜택
- 한글판 Pharmaceutical Engineering 열람 및 다운로드
- 온라인 & 오프라인 교육비 40% 할인
- 동영상 교육자료 무료시청
* 가입방법: 홈페이지(www.ispe.or.kr) - 회원가입 - 교육회원 - 신청서 작성 - 회원가입비 납부
* 회원가입비: 연25만원
- 신용카드 결재 및 무통장입금(세금계산서 발급)
-
PHARMACEUTICAL ENGINEERING (한글판)
-
May-June 2022 / Volume 42, Number 3
- VIRAL VECTOR PLATFORMS: Intersection of Facility and Program
- ATMP FACILITIES: Adapting to a Multimodal Future
- REIMAGINING CPV for a Pharma 4.0 World
- PANDEMIC PROGRESS: Industry's Journey From 2020 to Today
- MOVING FROM CLEANROOM to Isolation Technology for ATMPs
- ACCELERATING BIOPHARMACEUTICAL VIRTUAL FATS IN A PANDEMIC
- MATHEMATICAL MODELS in Experimental Design and Scale-Up
* 국제회원, 교육회원: www.ispe.or.kr - 출판물 - 매거진에서 확인 가능합니다.
-
Upcoming Conferences
2022 ISPE Biotechnology Conference
28-30 June 2022
Boston, MA USA
2022 ISPE China Spring Conference
25-27 August 2022
Hangzhou shi Zhejiang Province, China
Theme: Leverage global knowledge, Enable China excellence
-
Guidance Documents
Good Practice Guide: Membrane-Based WFI Systems
Published: May 2022
Pages: 196
Member Price: $295.00
Non-Member Price: $595.00
Good Practice Guide: Continuous Manufacturing of Oral solid Dosage Forms
Published: March 2022
Pages: 142
Member Price: $295.00
Non-Member Price: $595.00
APQ Guide: Change Management(CM) System
Published: February 2022
Pages: 152
Member Price: $215.00
Non-Member Price: $515.00
-
Good Practice Guide: Good Engineering Practice 2nd Edition
Published: October 2021
Pages: 244
Member Price: $295.00
Non-Member Price: $595.00
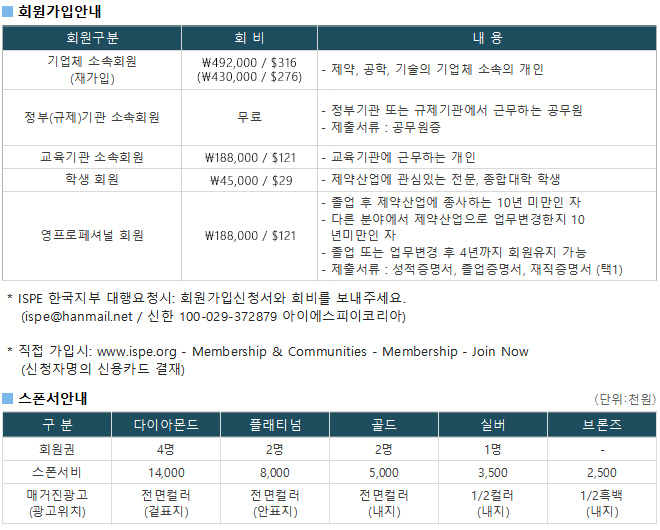
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
