-
게시판-관련소식
뉴스레터 2023년 10월호
2023-11-16 by ISPE Korea
-
MFDS 소식
의약품 GMP적합판정 및 적합판정서 발급 관련 업무처리방안(공무원지침서)개정알림
의약품 GMP적합판정서 기재방법을 EU등 국제기준과 조화하여 명확히 하기 위한 「의약품 GMP 적합판정 및 적합판정서 발급 관련 업무처리방안」(공무원지침서)를 붙임과 같이 개정하였음을 알려드립니다.
- 의료제품 개발 상담 사례집(의약품, 바이오의약품, 의료기기) 개정(2개정)
의료제품의 시행착오를 줄이고 신속한 제품화 지원을 위해 제품화지원팀에서 수행한 의약품, 바이오의약품, 의료기기 분야의 주요 상담 사례를 정리하여 발간하여 공유하오니 업무에 많은 활용이 되길 바랍니다.
-
-
GMP 가이드라인
-
[FDA] Policy for Testing of Alcohol (Ethanol) and Isopropyl Alcohol for Methanol
-
The guidance is intended to alert pharmaceutical manufacturers and pharmacists in Statelicensed pharmacies or Federal facilities who engage in drug compounding to the potential public health hazard of alcohol (ethyl alcohol or ethanol) or isopropyl alcohol contaminated with or substituted with methanol. During the Coronavirus Disease 2019 (COVID-19) public health emergency (PHE), the Food and Drug Administration (FDA) became aware of reports of fatal methanol poisoning of consumers who ingested alcohol-based hand sanitizer products that were manufactured with methanol or methanol-contaminated ethanol.
-
[PIC/S] GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS ANNEXES
-
The manufacture of sterile products covers a wide range of sterile product types (active substance, excipient, primary packaging material and finished dosage form), packed sizes (single unit to multiple units), processes (from highly automated systems to manual processes) and technologies (e.g. biotechnology, classical small molecule manufacturing systems and closed systems), This Annex provides general guidance that should be used in the design and control of facilities, equipment, systems and procedures used for the manufacture of all sterile products applying the principles of Quality Risk Management (QRM), to ensure that microbial, particulate and endocoxin/pyrogen contamination is prevented in the final product.
-
-
ISPE 국내외 소식
[한글판] PHARMACEUTICAL ENGINEERING
September-October 2023 / Volume 43, Number 5
- AN EVALUATION OF Postapproval CMC Change Timelines
- AIR SPEED QUALIFICATION: At Working Position or Working Level?
- CONSIDERATIONS FOR A Decentralized Manufacturing Paradigm
- NEW EU AI REGULATION AND GAMP®5
- 2023 ISPE Aseptic Conference Regulatory Panel
- INDUSTRY PANEL ON ANNEX1 IMPLEMENTATION STRATEGIES
* 국제회원 및 교육회원에게 10월 발송완료
* ISPE KOREA 홈페이지-출판물-매거진에서도 확인하실수 있습니다.
ISPE KOREA 교육회원 모집
* 가입대상: 제약 및 Biopharma 관련업계 종사하는 모든 분
* 회원혜택
- 한글판 Pharmaceutical Engineering 열람 및 다운로드
- 온라인 & 오프라인 교육비 40% 할인
- 동영상 교육자료 무료시청
* 가입방법: 홈페이지(www.ispe.or.kr) - 회원가입 - 교육회원 - 신청서 작성 - 회원가입비 납부
* 회원가입비: 연 25만원
-
Upcoming Conferences
2023 ISPE Pharma 4.0 & Annex 1 Conference
11-12 December 2023
Barcelona, Spain and Virtual
2024 ISPE Facilities of the Future Conference
29-30 January 2024
San Francisco, CA USA and Virtual
-
Guidance Documents
Good Practice Guide: Process Gases 2nd Edition
Published: October 2023
Pages: 152
Member Price: $295.00
Non-Member Price: $595.00
Guide: 503B Compounding
Published: August 2023
Page: 122
Member Price: $295.00
Non-Member Price: $595.00
Good Practice Guide: Containment for Potent Compounds
Published: December 2022
Pages: 258
Member Price: $295.00
Non-Member price: $595.00
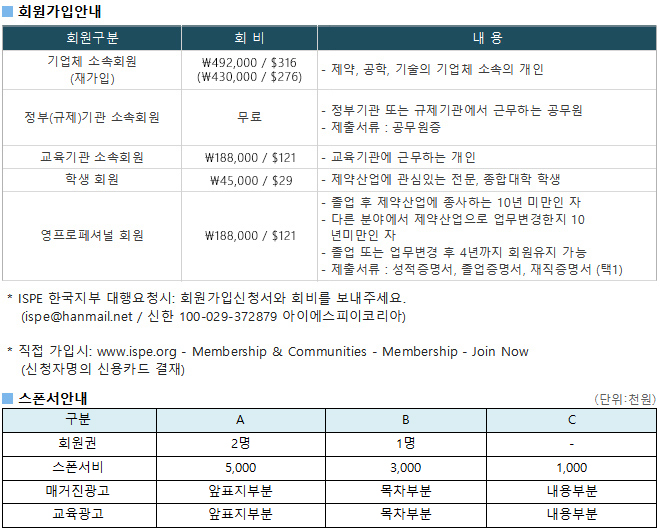
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
