-
게시판-관련소식
뉴스레터 2024년 5월호
2024-05-21 by ISPE Korea
-
MFDS 소식
바이오의약품 사전 GMP 평가지침 개정
바이오의약품 허가신청시 제조 및 품질관리기준(GMP) 실시상황 평가 절차와 관련하여 현재 운영중인 업무처리 절차를 개선하기 위하여 붙임과 같이 '바이오의약품 사전 GMP 평가지침(공무원지침서)을 개정하였음을 알려드리니 업무에 참고하시기 바랍니다.
- 식품의약품분야 시험검사기관 평가에 관한 규정 일부개정고시
식품의약품안전처 고시 제2024-21호
「식품·의약품분야 시험·검사등에 관한 법률」 제6조제4항 및 제16조제1항, 제2항에 따른 「식품·의약품분야 시험 검사기관 평가에 관한 규정」을 다음과 같이 개정 고시합니다.
-
-
GMP 가이드라인
-
[FDA] CVM GFI #253 Current Good Manufacturing Practice for Animal Cells, Tissues, and Cell-and Tissue-Based Products
-
The FDA's Center for Veterinary Medicine (CVM) is issuing this guidance to provide establishment that manufacture animal cells, tissues, and cell-and tissue-based products (ACTPs) with recommendations for meeting current good manufcturing practice (CGMP) requirements. All new animal drugs, including ACTPs, must be manufactured in accordance with CGMP to ensure that such drugs meet the requirements of the Federal Food, Drug, and Cosmetic Act (FD&C Act) as to safety, and have the identity, strength, quality, and purity characteristics which they purport to or represented to possess.
-
[PIC/S] GUIDANCE ON CLASSIFICAION OF GMP DEFICIENCIES
-
This guidance is inended to provide a tool to support the risk based classification of GMP deficiencies from inspections and to establish consistency amngst inspectorates.
This guidance will enable Industry to be informed of the pinciples used to classify GMP deficiencies and also provide examples of the classification of different types of deficiencies.
-
-
ISPE 국내외 소식
교육안내
[5.23] Extractables and Leachables Study
- 일시: 2024.05.23(목) 9:00~16:00
- 강사: 우성환, 강승훈(큐비디)
- 장소: 판교글로벌 R&D센터 A동 5층
- 대면(집합) 교육, 신청 접수중
[한글판] PHARMACEUTICAL ENGINEERING
May-June 2024 / Volume 44, Number 3
- POST-APPROVAL CHANGE MANAGEMENT for Cell and Gene Therapy Products
- BUILDING BETTER THERAPIES with Antibody Engineering
- CELL CULTUR MEDIA NANUFACTURING CONTROLS for Bio-Manufacturing
- QUALITY RISK MANAGENENT for Biopharmaceuticals
* 한글판 번역본은 6월중 국제회원 및 교육회원에게 정기 발송해 드립니다.
* ISPE KOREA 홈페이지-출판물-매거진에서도 확인하실수 있습니다.
ISPE KOREA 교육회원 모집
* 가입대상: 제약 및 Biopharma 관련업계 종사하는 모든 분
* 회원혜택
- 한글판 Pharmaceutical Engineering 열람 및 다운로드
- 온라인 & 오프라인 교육비 40% 할인
- 동영상 교육자료 무료시청
* 가입방법: 홈페이지(www.ispe.or.kr) - 회원가입 - 교육회원 - 신청서 작성 - 회원가입비 납부
* 회원가입비: 연 25만원
-
Upcoming Conferences
2024 ISPE Biotechnology Conference
16-18 June 2024
Boston, MA USA and Virtual
2024 ISPE Annual Meeting & Expo
13-16 October 2024
Orlando, FL USA and Virtual
2024 ISPE Pharma 4.0 and Annex 1 Conference
10-11 December 2024
Rome, Italy and Virtual
-
Guidance Documents
Guide: ATMPs - rAAV Comparability & Lifecycle Mgmt
Published: January 2024
Page: 98
Member Price: $395.00
Non-Member Price: $695.00
Guide: ATMPs - Allogeneic Cell Therapy
Published: January 2024
Page: 144
Member Price: $395.00
Non-Member Price: $695.00
Baseline Guide Vol 8: Pharma 4.0 1st Edition
Published: December 2023
Page: 270
Member Price: $295.00
Non-Member Price: $595.00
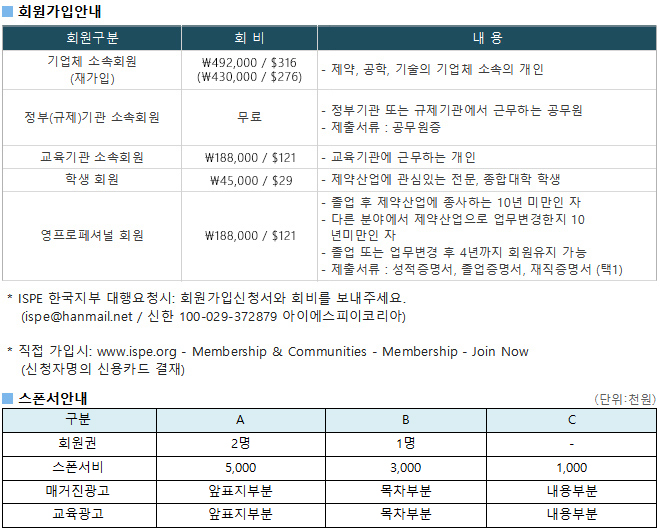
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
