-
게시판-관련소식
뉴스레터 2024년 12월호
2025-01-24 by ISPE Korea
-
MFDS 소식
바이오의약품 전문수탁 제조업체 GMP 평가절차(공무원지침서) 개정
바이오의약품 전문수탁 제조업체 GMP 적합판정 제도를 보다 합리적으로 운영하기 위해 '바이오의약품 전문수탁 제조업체 GMP평가절차(공무원지침서)'를 개정하였음을 알려드리니 업무에 참고하시기 바랍니다.
- 「의약품 표준제조기준」 일부개정고시(안) 행정예고 알림
의약품 표준제조기준 중 새로운 제형, 신규 배합성분의 추가 등을 통해 일반의약품을 활성화하여 소비자의 일반의약품 선택 폭을 확대하고자 「의약품 표준제조기준」 (식약처 고시) 일부 개정고시안을 붙임2과 같이 마련하여 행정예고하였음을 알려드립니다.
-
-
GMP 가이드라인
-
Current Good Manufacturing Practice-Guidance for Human Drug Compounding Outsourcing Facilities Under Section 503B of the FD&C Act Guidance for Industry
-
This guidance describes FDA's policies regarding compliance with current good manufacturing practice (CGMP) requirements for facilities that compound human drugs and register with FDA as outsourcing facilities under section 503B of the Federal Food, Drug, and Cosmetic Act (FD&C Act). Under section 501(a)(2)(B) of the FD&C Act, a drug is deemed to be adulterated if it is not produced in accordance with CGMP.
-
[PIC/S] Recommendation on Risk-Based Inspection Planning
-
This PIC/S Recommendation sets out a simple and flexible Quality Risk Management tool that may be used by Inspectorates when planning the frequency and scope of GMP. It is a methodology that is based upon the concept of rating manufacturing sites on the basis of an estimated risk that they may pose to patients, consumers, animals and users of medicines. The methodology also takes into account the risk to product quality.
-
-
ISPE 국내외 소식
[한글판] PHARMACEUTICAL ENGINEERING
November-December 2024 / Volume 44, Number 6
- 3R Initiative Within Roche's QC Network
- Decarbonizing Pharmaceutical Manufacturing Facilities
- Cloud Service Provider
* 한글판 번역본은 12월중 국제회원 및 교육회원에게 정기 발송해 드립니다.
* ISPE KOREA 홈페이지-출판물-매거진에서도 확인하실수 있습니다.
ISPE KOREA 교육회원 모집
* 가입대상: 제약 및 Biopharma 관련업계 종사하는 모든 분
* 회원혜택
- 한글판 Pharmaceutical Engineering 열람 및 다운로드
- 온라인 & 오프라인 교육비 40% 할인
- 동영상 교육자료 무료시청
* 가입방법: 홈페이지(www.ispe.or.kr) - 회원가입 - 교육회원 - 신청서 작성 - 회원가입비 납부
* 회원가입비: 연 25만원
-
Upcoming Conferences
2024 ISPE Pharma 4.0 and Annex 1 Conference
10-11 December 2024
Rome, Italy and Virtual
2025 ISPE Facilities of the Future Conference
27-28 January 2025
San Francisco, CA USA and Virtual
-
Guidance Documents
Good Practice Guide: Ozone Sanitization of Pharm Water Storage & Distribution Systems 2nd Edition
Published: October 2024
Page: 156
Member Price: $295.00
Non-Member Price: $670.00
Good Practice Guide: Heating, Ventilation, & Air Conditioning (Second Edition)
Published: September 2024
Page: 272
Member Price: $295.00
Non-Member Price: $670.00
Good Practice Guide: Unique ID of Glass Primary Containers
Published: May 2024
Page: 86
Member Price: $295.00
Non-Member Price: $595.00
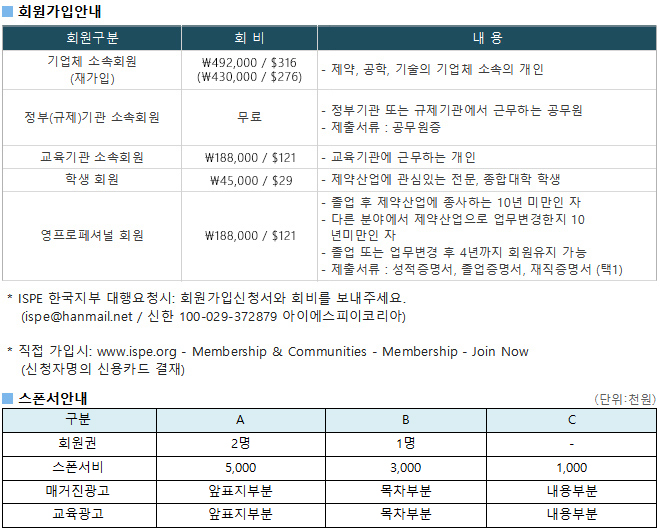
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
