-
게시판-관련소식
뉴스레터 2025년 1월호
2025-01-24 by ISPE Korea
-
MFDS 소식
「의약품 제조 및 품질관리에 관한 규정」 일부개정고시
의약품 GMP평가 및 적합판정 관리체제 개선 등 의약품 GMP제조관련 규제혁신을 위해 「의약품 제조 및 품질관리에 관한 규정」(식약처 고시)을 개정고시 하였음을 알려드립니다.
- 2024년 하반기 의약품 심사설명회 발표자료
제네릭의약품 품질심사과정에서 빈번히 발생하는 보완사항을 사례중심으로 소개하여 효율적 심사자료 준비방안을 제공하고자 함
제네릭의약품 품질심사 주요 보완사례집(민원인 안내서) 제정('24.10.30)
-
-
GMP 가이드라인
-
[FDA] Data Integrity and Compliance With Drug CGMP: Questions and Answers
-
The purpose of this guidance is to clarify the role of data integrity in current good manufacturing practice (CGMP) for drugs, as required in 21 CFR parts 210, 211, and 212. Unless otherwise noted, the term CGMP in this guidance refers to CGMPs for drugs (including biologics). FDA’s authority for CGMP comes from section 501(a)(2)(B) of the Federal Food, Drug, and Cosmetic Act (FD&C Act).
-
[PIC/S] 2023 Annual Report
-
The Pharmaceutical Inspection Co-operation Scheme (PIC/S) was established in 1995 as an extension to the Pharmaceutical Inspection Convention (PIC) of 1970 (see Annex 1). PIC/S is a non-binding co-operative arrangement between Regulatory Authorities in the field of Good Manufacturing Practice (GMP) of medicinal products for human or veterinary use.
-
-
ISPE 국내외 소식
[한글판] PHARMACEUTICAL ENGINEERING
January/February 2025 / Volume 45, Number 1
- A GMP Approach to Computerized System Life Cycle and IT Process Records
- REMOVING THE FRUSTRATION from Functional Risk Management
- REEVALUATING TRANSFER OF RTU Containers into Grade A
* 한글판 번역본은 2월중 국제회원 및 교육회원에게 정기 발송해 드립니다.
* ISPE KOREA 홈페이지-출판물-매거진에서도 확인하실수 있습니다.
ISPE KOREA 교육회원 모집
* 가입대상: 제약 및 Biopharma 관련업계 종사하는 모든 분
* 회원혜택
- 한글판 Pharmaceutical Engineering 열람 및 다운로드
- 온라인 & 오프라인 교육비 40% 할인
- 동영상 교육자료 무료시청
* 가입방법: 홈페이지(www.ispe.or.kr) - 회원가입 - 교육회원 - 신청서 작성 - 회원가입비 납부
* 회원가입비: 연 25만원
-
Upcoming Conferences
2025 ISPE Facilities of the Future Conference
27-28 January 2025
San Francisco, CA USA and Virtual
2025 ISPE Aseptic Conference
17-18 March 2025
Washington D.C., USA and Virtual
2025 ISPE Europe Annual Conference
12-14 May 2025
London, United Kingdom and Virtual
-
Guidance Documents
Good Practice Guide: SMEPAC-Standardized Methodology for the Evaluation of Pharma Airborne Particle Emissions from Containment Systems 3rd Edition
Published: December 2024
Page: 122
Member Price: $295.00
Non-Member Price: $670.00
Good Practice Guide: Ozone Sanitization of Pharm Water Storage & Distribution Systems 2nd Edition
Published: October 2024
Page: 156
Member Price: $295.00
Non-Member Price: $670.00
Good Practice Guide: Heating, Ventilation, & Air Conditioning (Second Edition)
Published: September 2024
Page: 272
Member Price: $295.00
Non-Member Price: $670.00
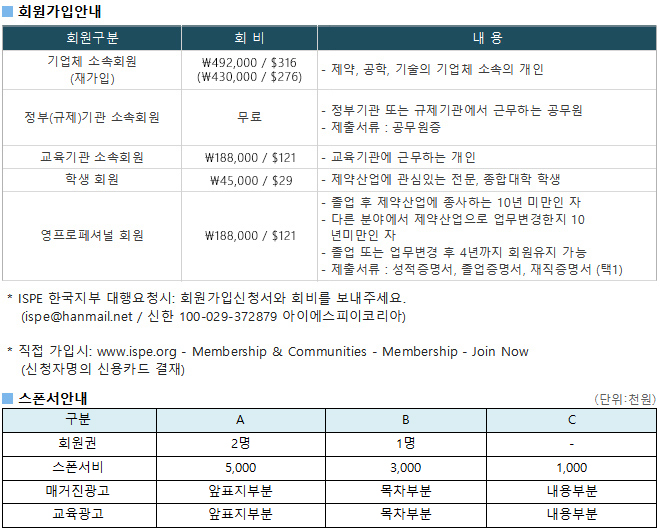
광고문의 TEL. 043-213-0442 E-MAIL. ispe@hanmail.net
ISPE Korea Affiliate | Office : 충북 청주시 흥덕구 직지대로 530, 702호 (송정동, 청주테크노S타워)
-
